Clinical Applications
Type II Diabetes
Type II diabetes, also called non-insulin-dependent diabetes mellitus (NIDDM), is characterized by high blood glucose resulting from the genetic defect of the GLUT4 genome. The latter is manifested in the diminished glucose uptake into skeletal muscles. In normal human beings, insulin combines with insulin receptors to change the membrane conformation of skeletal muscle fibers, allowing blood glucose to move down its concentration gradient into the fibers for metabolism. A disorder called ‘insulin resistance’ exists in Type II diabetic patients in which normal or even elevated levels of plasma insulin would not elicit normal glucose uptake into the muscle fibers. This article hypothesizes that these diabetic fibers exhibit less insulin receptors, or that these receptors exhibit abnormal molecular conformation, or both. Considering that highly metabolic muscle fibers constitute more than 50% of the human body by volume and weight, failure of blood glucose to gain entry would undoubtedly lead to high blood glucose and result in various organ failures sequentially. This is not the only defect, but it is likely the primary and significant one.
A potential genetic treatment of the disease involves myoblast transfer therapy (MTT) which is a platform technology of cell transplantation, genome therapy and tissue engineering.It consists of culturing immature muscle cells called myoblasts, derived originally from a 2g skeletal muscle biopsy from a healthy, young, male donor, and implanting them into the major muscle groups of the upper and lower extremities of the diabetic patients. The myoblasts exhibit natural cell fusion, and transfer their nuclei carrying the normal human genome into the host skeletal muscle fibers to effect genetic repair. Others fuse among themselves to form new myofibers that exhibit normal insulin receptors of donor origin. Through both mechanisms, new insulin receptors of donor origin that are genetically normal, will be produced in the skeletal myofibers of the host.
The survival, development, and functioning of the implanted allogeneic myoblasts have previously been demonstrated in studies involving about 240 muscular dystrophy subjects and two chronically myocardial infracted heart subjects with 100% safety and substantial efficacy results. Immunorejection was minimized using two months of cyclosporine following MTT. In addition, over 120 ischemic heart patients have received autologous myoblasts in their hearts in 10 countries. Mortality rate has been less than 10% traversing the last four years, with efficacy data being collected in Phase II clinical trials in Europe. Reported here are the world’s first genetic transplants of two Type II diabetic patients using allogeneic myoblasts.
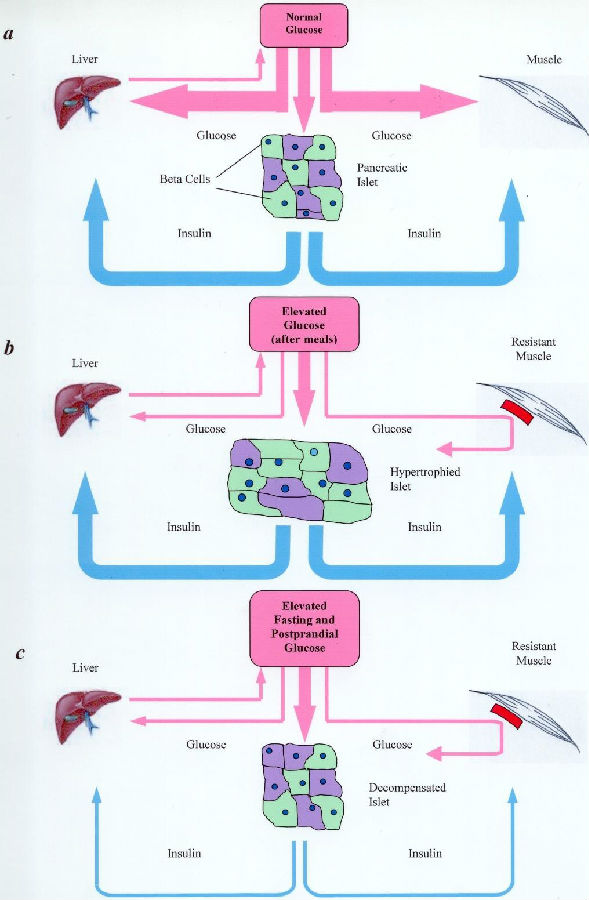
Type II diabetic myofibers
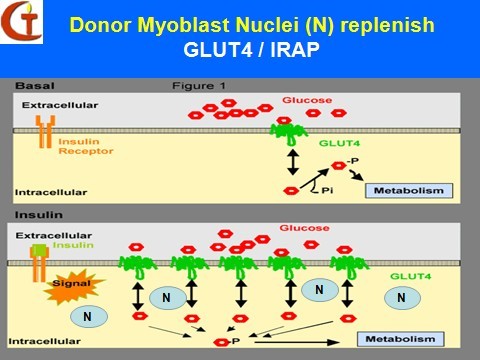
Donor Myoblasts Nuclei (N) replenish